EZYPOR®
Description :
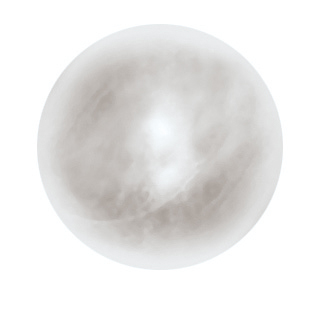
EZYPOR® is FCI’s ultimate high density polyethylene orbital implant with an innovative suturing platform featuring a smooth anterior surface and eight suture* tunnels. EZYPOR® is gently inserted into the eye socket with a sphere introducer (S6.3050). The use of the FCI sizer set (S6.3060) to select the appropriate implant diameter is recommended for the best cosmetic result.
Main characteristics :
• Indicated for enucleation, evisceration, and secondary implantation
• Made of Ultra High Molecular Weight PolyEthylene (UHMWPE)
• Porosity between 40 and 60% for optimum colonization of fibrovascular tissues
• Smooth anterior suturing platform with 8 suture tunnels to facilitate needle insertion and muscles fixation
• No need for autologous graft
• Sterile
*EZYPOR® 12 mm and 14 mm do not have a suturing platform.