BIKA® for DCR
Description :
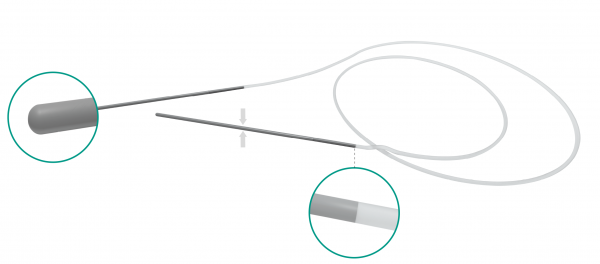
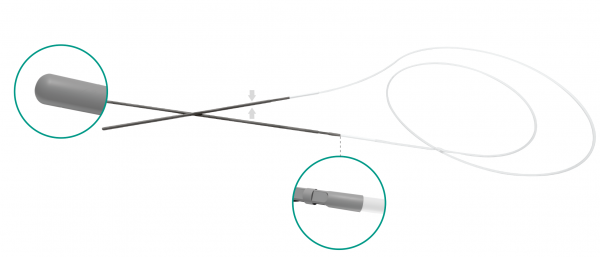
FCI BIKA® intubation systems are the first models designed for bi-canalicular intubation, especially in case of congenital nasolacrimal duct obstruction and dacryocystorhinostomy (DCR). The metallic probes are securely connected at the silicone tube for an easy and safe retrieval from the nose.
Main characteristics :
Designed by J.-A. Bernard, M.D.
- Indicated for dacryocystorhinostomy (DCR)
- Unique crimping process provides stronger connection between the tube and metal probe, virtually eliminating risk of separation during procedure
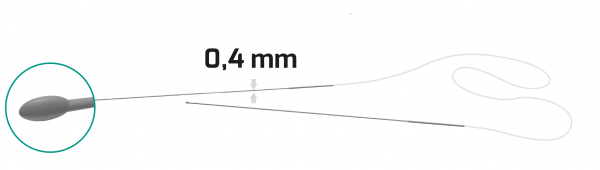
- 0.94 mm silicone tube / 53 mm long & 0.8 mm diameter metallic probe
- Indicated for dacryocystorhinostomy (DCR)
- Medical grade silicone
- Sterile